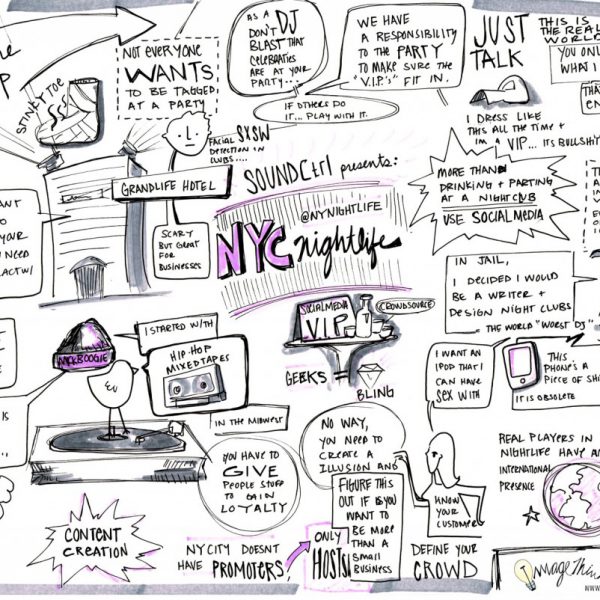
ImageThink is attending Social Media Week (well…at least 1/2 of Social Media Week!) Social Media Week offers a series virtually connecting conversations and activities around the world on emerging trends in social and mobile media across major industries. Some of the tracks that we will be taking visual notes at here in NYC are: Health and Wellness, Technology & Innovation, Social & Environmental Change, Education & Learning.
Yesterday, Heather created graphic summaries conversation when Tom Chernaik from CMP.LY and Marc Monseau lead group discussions at the Health & Wellness Content Hub at Saatchi & Saatchi Wellness, here in New York. Participants broke up into small groups to parallel process on what the FDA’s social media guidelines should be, as well as comments for the Physician Payment Sunshine Act. Heather, captured the highlights and key ideas in the following visual summary.
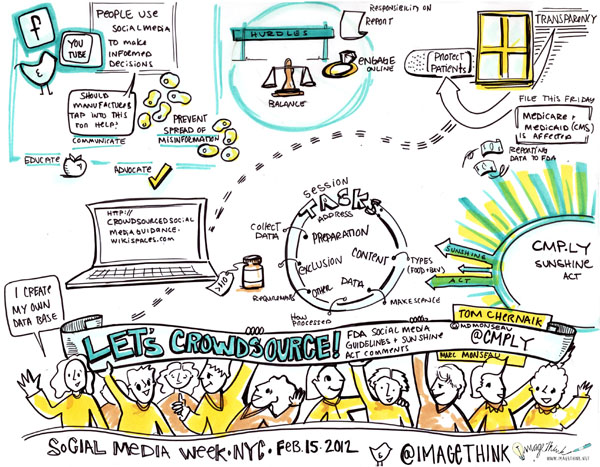
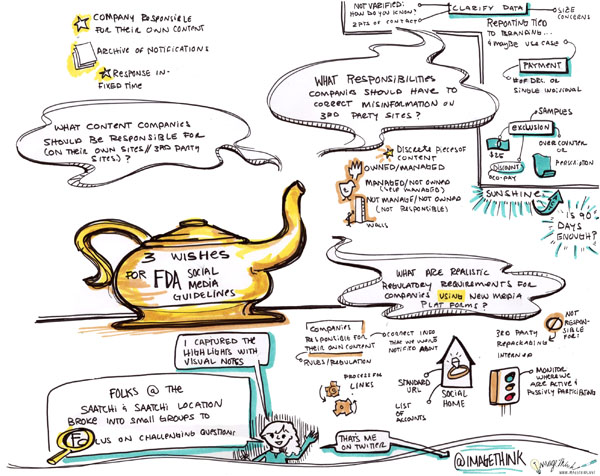
Here is a bit of information (thank you SMWHealth) about the FDA Social Media Guidelines: Since the advent of social media, The pharmaceutical firms, their agencies, and technology partners have eagerly awaited firm guidance form the FDA on what is permitted in terms of marketing online, and specifically social media. Earlier this year — and a full two years following hearings — the FDA issued draft guidance on “responding to unsolicited requests for off-label information about prescription drugs and medical devices,” or in other words, how pharmaceutical companies can engage with patients who discuss off-label matters online.
About the Sunshine Act: This proposed rule would require applicable manufacturers of drugs, devices, biologicals, or medical supplies covered by Medicare, Medicaid or the Children’s Health Insurance Program (CHIP) to report annually to the Secretary certain payments or transfers of value provided to physicians or teaching hospitals (“covered recipients”). In addition, applicable manufacturers and applicable group purchasing organizations (GPOs) are required to report annually certain physician ownership or investment interests. The Secretary is required to publish applicable manufacturers and applicable GPOs’ submitted payment and ownership information on a public Web site.